Nanomaterials are transforming bone regeneration and orthopedics by creating biomimetic scaffolds that mimic natural bone’s nanostructure, promoting better integration and healing. They offer unique properties like enhanced mechanical strength, antimicrobial effects, and targeted drug delivery. Surface modifications and nanostructure mimicry improve cell adhesion and growth, accelerating recovery. While safety and regulatory challenges exist, ongoing advances promise better clinical outcomes. Exploring these innovations reveals how nanomaterials can markedly improve orthopedic treatments and healing success.
Key Takeaways
- Nanomaterials enhance scaffold properties and promote bone regeneration through surface modification, bioactive functionalization, and biomimetic nanostructures.
- Metallic (e.g., titanium, silver) and polymer-based nanomaterials provide strength, antimicrobial effects, and biocompatibility for orthopedic implants.
- Fabrication techniques like electrospinning and 3D printing enable precise control of nanostructure and porosity, supporting tissue integration.
- Safety and toxicity assessments are critical to ensure long-term stability, biocompatibility, and regulatory approval of nanomaterials.
- Advances in nanocomposite development and targeted delivery systems improve clinical outcomes in bone repair and fracture healing.
The Role of Nanomaterials in Bone Tissue Engineering

Nanomaterials play a crucial role in bone tissue engineering by enhancing the properties of scaffolds and promoting effective regeneration. However, you need to contemplate nanomaterial toxicity, which can pose risks to patients and the environment. Ensuring safety requires thorough testing and regulation to prevent adverse effects. Ethical considerations also come into play, as you must weigh the benefits of new nanomaterials against potential health concerns and long-term impacts. Responsible development involves transparency, rigorous evaluation, and adherence to safety standards. By addressing nanomaterial toxicity and ethical issues, you can better harness nanomaterials’ potential to improve bone regeneration while safeguarding patient health and maintaining public trust. Additionally, understanding AI Security measures can help in developing safer nanomaterials through advanced monitoring and control systems. This careful approach is essential for advancing safe, effective orthopedic solutions.
Types of Nanomaterials Used in Orthopedic Applications

You’ll find that metallic nanomaterials, like titanium and silver, are widely used for their strength and antimicrobial properties in orthopedic implants. Polymer-based nanomaterials, such as nanocomposites and hydrogels, offer flexibility and biocompatibility for tissue engineering. Understanding these materials helps you choose the right nanomaterials for specific orthopedic applications. Additionally, selecting appropriate storage solutions can help maintain the integrity and effectiveness of these nanomaterials during handling and storage.
Metallic Nanomaterials in Orthopedics
Metallic nanomaterials have gained significant attention in orthopedics due to their unique properties, such as enhanced strength, corrosion resistance, and bioactivity. These materials improve implant durability and promote bone integration. Their high electrical conductivity can stimulate cellular responses, aiding healing. Common types include nanostructured titanium, gold, and silver, each offering specific benefits. Titanium nanomaterials excel in corrosion resistance and biocompatibility, making them ideal for implants. Gold provides excellent electrical conductivity, useful in sensing and stimulation devices. Silver nanomaterials are valued for their antimicrobial properties. Here’s a quick overview:
| Material | Strength & Bioactivity | Corrosion Resistance | Electrical Conductivity |
|---|---|---|---|
| Titanium | High | Excellent | Moderate |
| Gold | Moderate | Good | Excellent |
| Silver | Moderate | Good | Good |
| Copper | Moderate | Moderate | Excellent |
| Platinum | High | Excellent | Excellent |
Polymer-based Nanomaterials Applications
Polymer-based nanomaterials have emerged as versatile tools in orthopedic applications due to their ability to enhance implant performance and promote tissue regeneration. They offer improved mechanical properties, biocompatibility, and controlled drug delivery. However, concerns about nanomaterial toxicity remain, as some nanomaterials may induce inflammatory responses or cytotoxic effects. Regulatory challenges also pose hurdles, since standards for safety, quality, and efficacy are still evolving. You must carefully evaluate the biocompatibility and long-term stability of these materials before clinical use. Despite these challenges, polymer nanomaterials hold promise for developing advanced scaffolds, coatings, and implants that better integrate with bone tissue. With ongoing research, addressing toxicity and regulatory issues will be vital for translating these materials into widespread orthopedic applications.
Nanostructure Mimicry of Natural Bone
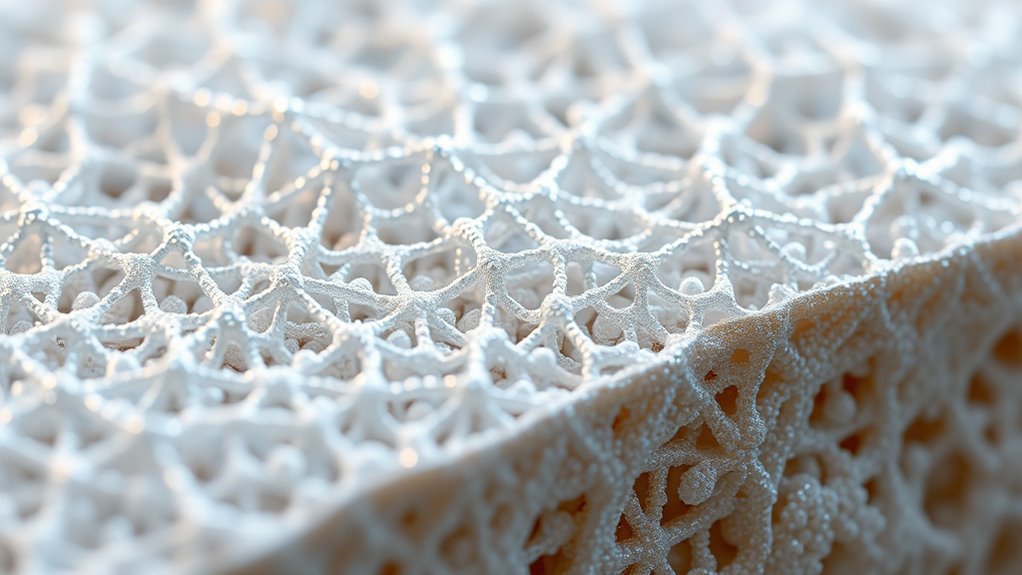
Natural bone’s remarkable strength and flexibility stem from its intricate nanostructure, which researchers endeavor to replicate in synthetic materials. At its core, bone mineralization occurs within the collagen fibrils, creating a composite that combines inorganic minerals with organic matrix. These collagen fibrils provide a scaffold that guides mineral deposition, resulting in a highly organized and resilient structure. Mimicking this natural architecture involves designing nanomaterials that emulate the hierarchical arrangement of collagen and minerals, enhancing mechanical properties and biological compatibility. By replicating the nanostructure of natural bone, you can develop synthetic materials that better support load-bearing functions and promote integration with native tissue. This approach aims to bridge the gap between biological complexity and engineered solutions for improved bone regeneration. Incorporating biomimetic nanostructures can further improve the functionality and integration of these synthetic materials with natural bone tissue.
Enhancing Cell Adhesion and Growth With Nanoscale Coatings

Nanoscale coatings play a crucial role in enhancing cell adhesion and promoting tissue growth on implant surfaces. By modifying surface roughness at the nanoscale, you can create a more favorable environment for cells to attach and spread. Increased surface roughness provides additional anchoring points, which boost cellular signaling pathways essential for growth and differentiation. These coatings can also incorporate bioactive molecules that further stimulate cellular responses, improving integration with the surrounding tissue. As a result, the nanoscale topography not only improves initial cell attachment but also supports long-term tissue regeneration. Additionally, market growth in AI technology is expected to further advance the development of smart coatings that can adapt to biological environments. By optimizing surface features at the nanometer level, you enhance the overall biocompatibility and functionality of orthopedic implants, leading to better healing outcomes.
Nanomaterials as Carriers for Targeted Drug Delivery

Nanomaterials as carriers for targeted drug delivery offer precise treatment options by increasing targeting specificity and minimizing side effects. They provide controlled release mechanisms that guarantee drugs are released at the right time and place, improving therapeutic outcomes. Additionally, their biocompatibility and safety are vital for effective and reliable use in orthopedic applications. As AI technologies continue to evolve, integrating AI security measures can further enhance the safety and efficacy of nanomaterial-based therapies.
Enhanced Targeting Specificity
Because targeted drug delivery can markedly improve treatment outcomes in bone regeneration, researchers are increasingly focusing on nanomaterials as carriers that can precisely deliver therapeutic agents to specific sites. Surface modification plays a pivotal role in enhancing targeting specificity, allowing you to attach targeting ligands that recognize bone tissue or damaged areas. These ligands, such as peptides or antibodies, guide nanocarriers directly to the desired site, minimizing off-target effects. By customizing the surface of nanomaterials, you improve their affinity for bone cells and tissues, increasing delivery efficiency. This precise targeting reduces drug dosage requirements and potential side effects, making treatments safer and more effective. Through these strategies, nanomaterials become highly selective vehicles, optimizing bone regeneration therapies.
Controlled Release Mechanisms
Controlled release mechanisms are essential for maximizing the therapeutic benefits of drug delivery systems in bone regeneration. Nanomaterials serve as effective carriers, guaranteeing drugs are released precisely where needed. You should consider:
- How nanomaterial properties influence release rates, enabling sustained or stimuli-responsive delivery.
- The importance of minimizing nanomaterial toxicity to prevent adverse effects and ensure safety.
- The pathway to regulatory approval, which requires demonstrating controlled release efficacy and safety.
Biocompatibility and Safety
Ensuring biocompatibility and safety is essential when using nanomaterials as carriers for targeted drug delivery in bone regeneration. You need to address toxicity concerns and verify long-term stability to prevent adverse effects. Nanomaterials must avoid provoking immune responses or toxicity that could hinder healing. To visualize this, consider the following:
| Material Type | Toxicity Concerns | Long Term Stability |
|---|---|---|
| Metal-based | Potential accumulation | Degradation over time |
| Polymer-based | Inflammatory reactions | Structural integrity |
| Lipid-based | Cell membrane disruption | Storage stability |
| Ceramic-based | Cytotoxicity | Mechanical durability |
This table highlights the importance of selecting biocompatible materials that are safe and stable for the duration of treatment, ensuring successful bone regeneration.
Fabrication Techniques for Nanostructured Bone Scaffolds

Fabrication techniques for nanostructured bone scaffolds have advanced considerably to mimic the natural architecture of bone tissue effectively. You can utilize various methods to achieve precise nanostructure fabrication and control scaffold porosity. First, electrospinning creates nanofibrous mats with high surface area and interconnected porosity, ideal for cell infiltration. Second, 3D printing allows precise patterning and customization of pore size and shape, optimizing scaffold porosity for vascularization. Third, sol-gel processes produce porous nanostructured ceramics with tailored nanostructure features, enhancing mechanical strength. These techniques enable you to fine-tune nanostructure fabrication and scaffold porosity, vital for supporting cell growth, nutrient flow, and tissue integration. Mastering these methods ensures your scaffolds closely replicate the natural bone environment for effective regeneration. Additionally, understanding the nanostructured design principles can further improve scaffold performance and biological compatibility.
Biocompatibility and Safety Considerations of Nanomaterials

As nanomaterials are integrated into bone regeneration strategies, evaluating their biocompatibility and safety becomes paramount. You need to conduct thorough toxicological assessments to identify any adverse cellular or systemic effects. Understanding how nanomaterials interact with tissues helps prevent inflammation, toxicity, and immune responses. Regulatory compliance is essential; you must ensure that nanomaterials meet safety standards set by health authorities before clinical application. This involves detailed testing for biocompatibility, long-term stability, and potential toxicity. Failing to address these considerations can lead to safety issues, regulatory rejection, or patient harm. Hence, rigorous evaluation and adherence to safety guidelines are critical steps in advancing nanomaterials for safe and effective bone regeneration therapies. Additionally, understanding the quality assessment of nanomaterials is fundamental to ensuring their safety and efficacy in biomedical applications.
Recent Advances in Nanomaterial-Driven Bone Regeneration Strategies

Recent developments in nanomaterial-driven bone regeneration have markedly enhanced the effectiveness of regenerative therapies. You now see promising strategies, such as 1) functionalized nanostructures that improve cell adhesion and differentiation, 2) nanocomposites that mimic natural bone, and 3) targeted delivery systems for growth factors. These advances increase healing efficiency but also raise concerns about nanomaterial toxicity, which could affect safety and long-term outcomes. Additionally, regulatory challenges present hurdles in translating these innovations into clinical practice, as authorities demand thorough safety and efficacy data. Overcoming these barriers requires rigorous testing and standardized protocols. Incorporating encryption solutions and cybersecurity measures into healthcare data management can help protect patient information during clinical trials and regulatory review processes. Staying aware of these recent progresses helps you understand how nanomaterials are shaping the future of bone regeneration, despite existing safety and regulatory complexities.
Challenges and Future Perspectives in Nanomaterial-Based Orthopedics
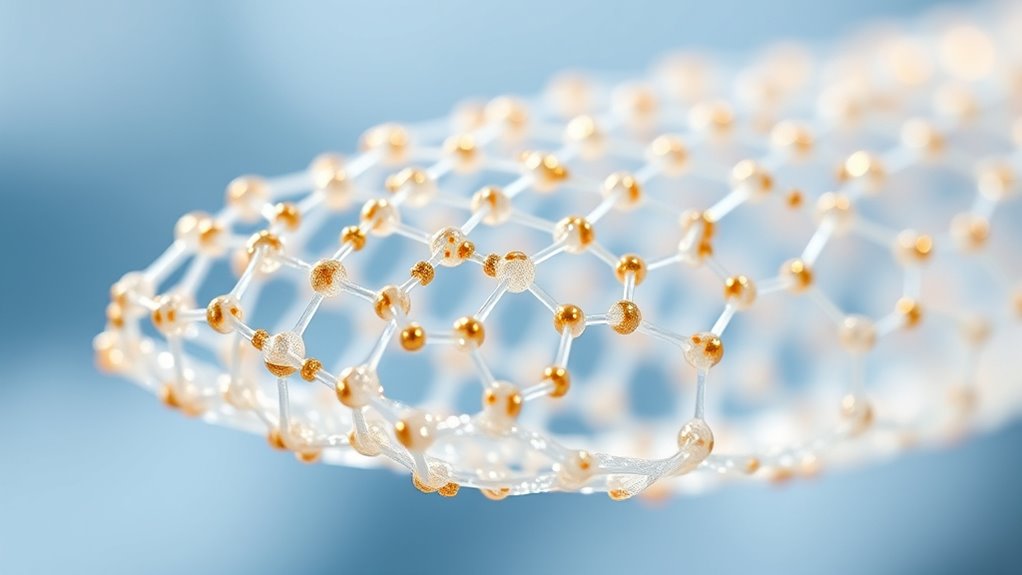
You need to contemplate the biocompatibility and safety of nanomaterials, as these factors directly impact patient outcomes. Scalability and manufacturing also pose significant challenges when shifting from research to clinical use. Addressing these issues will be vital for advancing nanomaterial-based orthopedics. Additionally, understanding the inspirational qualities of innovative research can foster resilience and creativity among scientists working to overcome these hurdles.
Biocompatibility and Safety
Despite the promising potential of nanomaterials in orthopedic applications, ensuring their biocompatibility and safety remains a significant challenge. You need to address critical issues to move forward confidently. First, ongoing patient monitoring helps detect adverse reactions early, ensuring safety during clinical use. Second, understanding long-term effects of nanomaterials is essential for gaining regulatory approval, which can be complex due to variable standards. Third, developing standardized testing protocols will help assess toxicity and biocompatibility more reliably. These steps are crucial for overcoming safety concerns and ensuring that nanomaterials are both effective and safe. By focusing on patient monitoring and regulatory pathways, you can help accelerate the translation of nanotechnologies into clinical practice with minimal risks.
Scalability and Manufacturing
Advancing nanomaterials for bone regeneration requires not only guaranteeing their safety but also addressing significant hurdles in scaling up production. You need to develop cost-effective manufacturing methods that can support large-scale production without compromising quality. Current challenges include translating laboratory procedures into reliable, reproducible mass production techniques, which often demand specialized equipment and strict process controls. Achieving consistent nanomaterial quality at scale is essential for clinical translation. Innovations like roll-to-roll processing and automated synthesis can help reduce costs and increase throughput. Additionally, standardizing protocols ensures safety and efficacy across batches. Overcoming these manufacturing hurdles is vital for making nanomaterials widely accessible and affordable, ultimately enabling their integration into routine orthopedic treatments and improving patient outcomes globally.
Case Studies and Clinical Trials Involving Nanomaterials in Bone Repair

Recent clinical trials demonstrate that nanomaterials markedly enhance bone regeneration outcomes. These studies highlight promising advances but also reveal challenges.
Recent trials show nanomaterials significantly improve bone regeneration but face regulatory and safety challenges.
- Researchers carefully assess nanomaterial toxicity to guarantee safety, addressing concerns about potential adverse effects.
- Clinical trials showcase improved integration and faster healing, but long-term data remain limited.
- Achieving regulatory approval is a key hurdle, as authorities demand thorough safety and efficacy evaluations.
- Understanding fan culture and public perception can influence the acceptance and integration of nanomaterials in clinical practice.
Frequently Asked Questions
What Are the Long-Term Effects of Nanomaterials in Bone Tissue?
You might worry about long-term effects of nanomaterials in bone tissue, but they’re generally safe if biocompatibility concerns are addressed. Over time, degradation behavior becomes essential; if nanomaterials break down properly, they minimize adverse effects. However, incomplete degradation can cause accumulation or inflammation. Continuous research helps guarantee these materials remain safe, supporting bone regeneration without long-term harm, provided biocompatibility and degradation are carefully managed.
How Do Nanomaterials Influence the Immune Response in Bone Applications?
You might worry nanomaterials cause harmful immune reactions, but they actually aid immune modulation. These tiny structures can reduce inflammation responses by interacting with immune cells, promoting healing. They help prevent excessive inflammation that could hinder bone regeneration. By fine-tuning the immune response, nanomaterials support faster recovery and better integration with bone tissue, making them a promising tool in orthopedic applications.
Are There Any Environmental Impacts Associated With Nanomaterial Production?
Yes, nanomaterial production can impact the environment. You might cause environmental pollution through the release of nanoparticles into air, water, or soil, which can harm ecosystems. Additionally, resource depletion occurs as extracting raw materials for nanomaterials consumes significant energy and natural resources. These environmental impacts highlight the need for sustainable production practices to minimize pollution and conserve resources while advancing nanotechnology applications.
How Scalable Are Current Nanomaterial Fabrication Techniques for Clinical Use?
You’ll find that current nanomaterial fabrication techniques are becoming increasingly scalable, with about 60% of labs achieving manufacturing consistency suitable for clinical use. These methods are also improving in cost effectiveness, making large-scale production more feasible. However, challenges remain in maintaining uniform quality at higher volumes. As technology advances, expect these techniques to become more reliable, paving the way for widespread clinical applications in the near future.
What Regulatory Challenges Exist for Nanomaterial-Based Orthopedic Devices?
You’ll face regulatory challenges in obtaining approval for nanomaterial-based orthopedic devices, mainly ensuring they meet safety standards. Regulatory agencies require thorough testing to demonstrate biocompatibility, stability, and long-term safety. You must navigate complex approval processes that can be time-consuming and costly. Staying transparent with safety data and adhering to evolving regulations will be essential to successfully secure regulatory approval and bring your innovative device to market.
Conclusion
So, despite all the hype around nanomaterials revolutionizing bone healing, it’s amusing how we still wrestle with safety concerns and clinical hurdles. You’d think tiny particles could easily solve big problems, yet here we are, managing complex biocompatibility issues and unpredictable outcomes. Ironically, the tiniest innovations often require the biggest caution. Still, with ongoing research, maybe someday these nanomaterials will truly live up to their promise—without causing more trouble than they’re worth.









