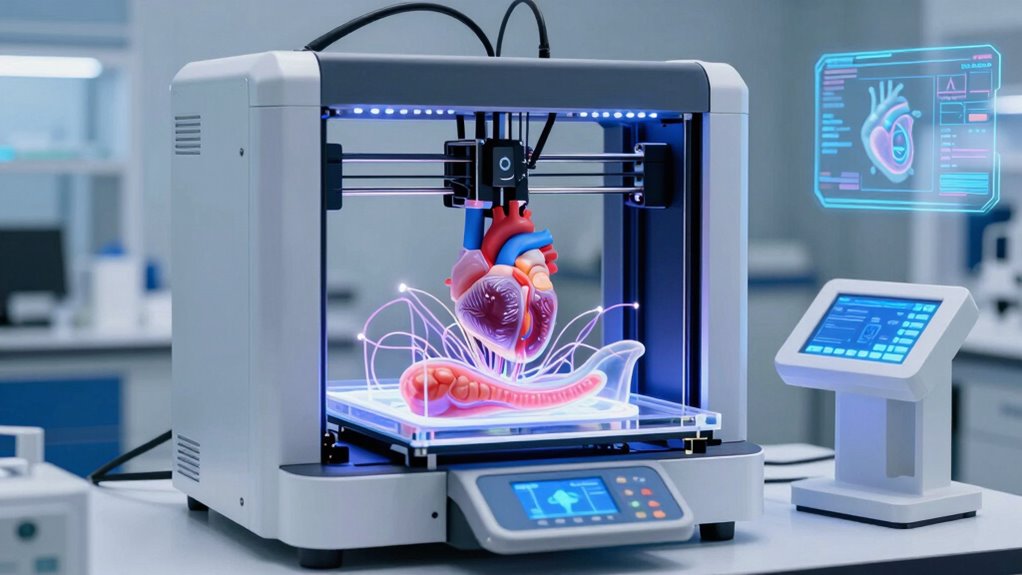
Nanotech is transforming organ printing by enabling ultra-precise microarchitecture, improved microvascular networks, and better integration of tissues. It allows you to create complex, functional organs with faster, more accurate methods, overcoming key challenges like vascularization and cell sourcing. As these innovations advance, manufacturing scalable, safe, and personalized transplants becomes more achievable. Stay with us to uncover how nanotech-driven bioprinting promises a future where organ rejection and shortages may become things of the past.
Key Takeaways
- Nanotechnology enhances bioprinting precision, enabling the creation of microarchitectures and microchannels essential for functional organ vasculature.
- Integration of nanomaterials improves scaffold strength, biocompatibility, and supports cellular growth and tissue maturation.
- Nanotech-based sensors and delivery systems facilitate real-time monitoring and targeted delivery of growth factors during organ development.
- Advances in nanomaterials enable the fabrication of more realistic, biodegradable, and immune-compatible biomaterials for transplantation.
- Combining nanotechnology with bioprinting accelerates organ maturation and may reduce rejection risks, advancing the future of transplant medicine.
Advances in Bioprinting Technologies and Techniques
Recent breakthroughs in bioprinting technologies have remarkably enhanced the speed, precision, and viability of tissue fabrication. You can now use volumetric and light-based methods to solidify entire 3D structures with patterned light, reducing fabrication time and minimizing cell damage. Embedded and one-pot resin approaches create sacrificial microchannels for vasculature, simplifying microcapillary network production. Multi-nozzle systems and spheroid-based setups allow you to place hundreds or thousands of cell aggregates simultaneously, increasing throughput and enabling larger, more complex tissues with high cell viability. Real-time imaging combined with AI-driven adaptive control helps identify cell positions during printing and automatically adjust parameters, improving reproducibility and reducing waste. UST projector rankings are also being explored to enhance tissue bioprinting environments by controlling ambient light conditions, further improving precision. The integration of bioprinting automation is streamlining the process and reducing human error, bringing us closer to clinical applications. Additionally, advances in bioprinting materials are expanding the range of tissue types that can be fabricated, making organ-scale printing more feasible. These advances are pushing bioprinting closer to producing functional, organ-scale tissues suitable for transplantation. Moreover, incorporating natural biomaterials can improve cell compatibility and tissue integration, facilitating more successful transplants. As the field progresses, precision control techniques are becoming increasingly vital for achieving the desired tissue architecture and functionality.
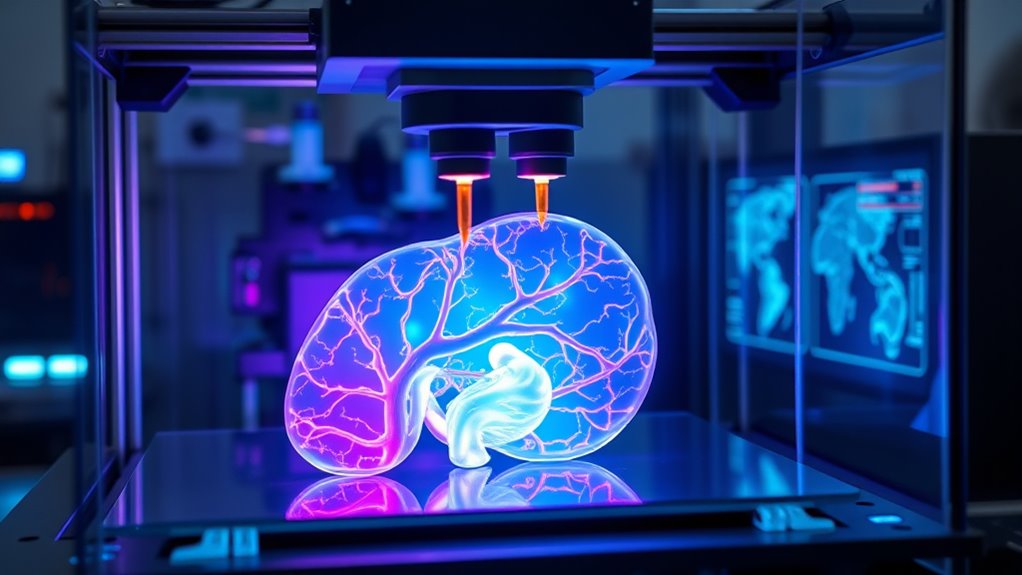
Overcoming Vascularization Challenges for Organ Survival
Creating functional, large-scale organs through bioprinting hinges on overcoming a critical hurdle: establishing a dense and reliable microvascular network that guarantees tissue survival. You need microcapillaries close enough to every cell, ensuring oxygen and nutrient delivery while removing waste. Recent innovations use sacrificial materials and dissolvable resins to create precise microchannels during printing, allowing scalable microvascular architectures. Biodegradable elastic hydrogels are designed to support these channels, gradually being replaced by native tissue. Computational vascular-tree algorithms accelerate design, enabling complex, organ-specific perfusion networks. While macrovasculature has been printed for perfusion, achieving capillary-level networks remains challenging. Advances in microfabrication continue to enhance the precision of creating tiny vascular channels, bringing us closer to fully functional bioprinted organs. Additionally, ongoing research into vascular regeneration techniques offers promising avenues for integrating printed microvessels with host tissue, further improving transplant success rates. Furthermore, understanding angiogenesis processes is crucial for developing successful integration strategies between printed vessels and the body’s existing vasculature.
Sourcing and Manufacturing Cells for Transplantation
Sourcing and manufacturing cells for transplantation pose significant challenges in bioprinting organs at scale. You need enough high-quality, functional cells—billions to trillions—to build a whole organ. Autologous cells, like patient-derived iPSCs, are ideal for immune compatibility but require lengthy reprogramming and expansion. Allogeneic or gene-edited cell lines can reduce production time but raise immunogenicity concerns. Manufacturing bottlenecks include maintaining cell phenotype during large-scale growth, ensuring quality control, and meeting safety standards. To clarify, here’s a breakdown:
| Cell Source | Advantages | Limitations |
|---|---|---|
| Autologous iPSCs | Immunocompatible, personalized | Time-consuming, costly, limited scale |
| Allogeneic Cells | Rapid, scalable, off-the-shelf | Immunogenicity, rejection risk |
| Gene-Edited Cells | Reduced rejection, tailored | Regulatory hurdles, safety concerns |
| Primary Cells | Mature, functional tissue | Limited expansion, donor variability |
| Stem Cell Banks | Ready availability, diverse | Compatibility, differentiation control |
Designing Biomaterials for Long-Term Integration
Designing biomaterials for long-term integration requires careful tuning of degradation rates, mechanical properties, and biological interactions to guarantee seamless tissue regeneration. You need materials that support cell attachment, migration, and differentiation while gradually degrading to make space for native tissue. Mechanical compliance matching the target organ prevents stress concentrations and ensures durability under physiological loads. Incorporate biochemical cues like growth factors to promote tissue-specific maturation and reduce immune responses. You must also consider immune acceptance; materials should minimize inflammation and chronic foreign body reactions. Using biodegradable hydrogels and composite scaffolds enables initial structural support while allowing tissue remodeling over months. Additionally, understanding the importance of safe installation practices and proper handling can help ensure that these biomaterials perform effectively within the body. Achieving this balance ensures your printed organ becomes a functional, integrated part of the body, capable of sustaining life long-term, especially when considering biocompatibility and long-term stability. Moreover, ongoing research into biodegradation kinetics helps optimize these materials for durable, functional integration, and advancements in material design continue to enhance their performance and compatibility. Recognizing the significance of immune modulation strategies can further improve integration outcomes and reduce rejection risks.
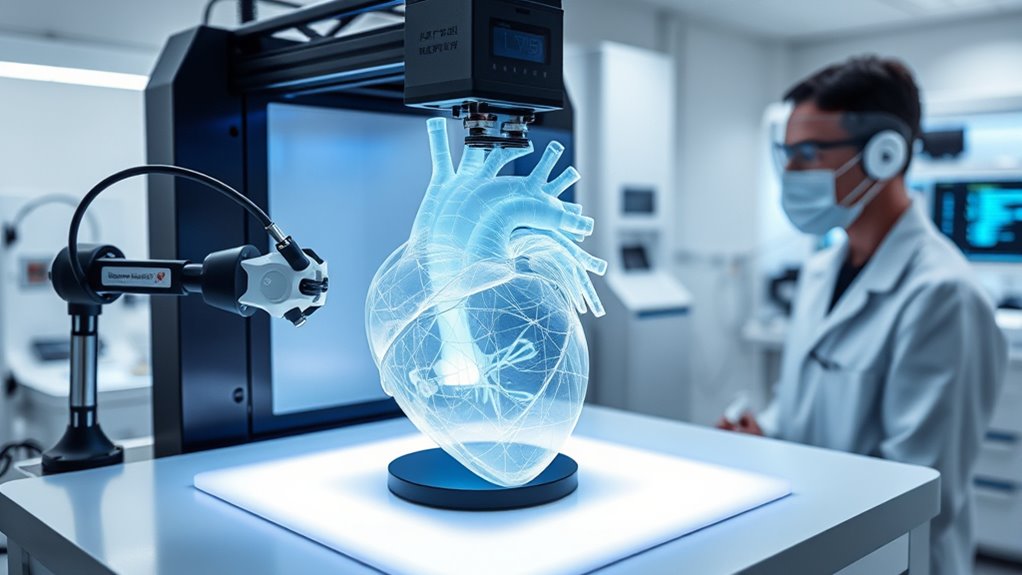
Achieving Functional Maturity in 3D-Printed Organs

To make certain printed organs function like native tissues, you need to focus on microarchitecture precision, such as replicating nephrons or cardiac pathways. Implementing maturation stimuli like mechanical forces, biochemical cues, and co-culture techniques can accelerate development and improve performance. Combining these strategies helps bridge the gap between initial fabrication and fully mature, functional organs ready for clinical use. Additionally, leveraging digital revolutions in bioprinting technology can further enhance the accuracy and efficiency of organ production.
Microarchitecture Precision
Achieving the true functional maturity of 3D-printed organs hinges on replicating their intricate microarchitecture with high precision. You need to accurately reproduce tiny structures like nephrons in kidneys, hepatic lobules in liver, or cardiac conduction pathways. Advanced bioprinting methods, such as light-based and volumetric techniques, enable rapid fabrication with fine resolution, ensuring microchannels and cellular arrangements match native tissues. Sacrificial resin approaches allow you to create microcapillaries essential for perfusion, while multi-nozzle systems deposit cell aggregates precisely, promoting complex tissue organization. Computational algorithms help design vascular networks that fit organ geometries, speeding up planning and printing. This microarchitecture fidelity is crucial for ensuring proper cell functionality, nutrient flow, and tissue integration—cornerstones for developing fully functional, transplant-ready organs. Additionally, leveraging Vetted electric bike conversion kits can inspire innovations in portable bioprinters by integrating lightweight, reliable power sources into mobile manufacturing units. Ensuring consistent microarchitecture quality requires rigorous quality control measures, which are vital for safe clinical applications. Incorporating microarchitecture precision techniques from connected home fitness technology can further enhance the accuracy and reliability of bioprinting processes.
Maturation Stimuli Strategies
Mature function in 3D-printed organs depends heavily on applying specific stimuli that mimic natural developmental cues. You can enhance organ maturation through mechanical, biochemical, and electrical signals, which guide cell differentiation and tissue organization. These stimuli encourage cells to develop native-like functionality, including vascularization, contractility, and metabolic activity. To clarify, consider this table:
| Stimulus Type | Purpose | Example Application |
|---|---|---|
| Mechanical | Promote structural integrity | Pulsatile flow in bioreactors |
| Biochemical | Drive cell signaling | Growth factors, cytokines |
| Electrical | Enhance functional activity | Cardiac pacing, electrical stimulation |
| Microarchitectural | Guide tissue organization | Scaffold patterning |
| Perfusion | Improve nutrient delivery | Dynamic flow systems |
Additionally, understanding the developmental cues involved in natural organ growth can inform the design of these stimuli, ensuring more effective maturation processes. Recognizing the importance of cell signaling pathways can further optimize the maturation process, leading to more functional transplanted organs. Incorporating bioreactor dynamics can help replicate physiological conditions more accurately. Moreover, employing mechanical stimulation techniques can significantly accelerate tissue development. Incorporating these strategies allows researchers to accelerate functional maturation, making printed organs viable for transplantation.
Pathways to Clinical Application and Ethical Considerations
Advances in bioprinting technologies are paving the way for clinical applications, yet translating these innovations into patient care involves steering complex regulatory and ethical landscapes. You’ll need to navigate evolving standards for safety, efficacy, and manufacturing quality, which are still under development. Regulatory agencies are working to create frameworks for combined products like cells, biomaterials, and printers, but approval processes remain lengthy. Establishing clear regulatory pathways is essential to streamline approval and ensure consistent standards across markets. Additionally, developing standardized manufacturing processes can help maintain quality and safety across different production sites. Ethically, you must consider equitable access, patient consent, and ownership of bioprinted organs. Balancing innovation with safety is critical to prevent misuse or unintended consequences. Public trust depends on transparent communication about risks and benefits. As you push toward clinical trials, you’ll also face challenges related to cost, scalability, and long-term monitoring, all of which influence the path from lab to bedside. To address these concerns, engaging with ethical frameworks can guide responsible development and deployment of bioprinting technologies. Furthermore, understanding the security zone of data involved in bioprinting can help protect patient information from cyber threats.
Frequently Asked Questions
How Close Are We to Fully 3d-Printed, Implantable Human Organs?
You’re still a few years away from fully 3D-printed, implantable human organs. While advances in bioprinting have produced functional tissue modules, complex organs like hearts and kidneys require precise microarchitecture, vascularization, and maturation. Clinical applications are expected first with smaller constructs like blood vessels or patches within the next 5-10 years. Full organs will need more breakthroughs in vascular networks, immune compatibility, and regulatory approval, likely taking a decade or more.
Can Bioprinted Organs Be Personalized for Immune Compatibility?
Yes, bioprinted organs can be personalized for immune compatibility. You can use autologous cells derived from your own tissues, which minimizes rejection risks. Researchers are also exploring allogeneic, universal-donor, and gene-edited cell lines to further reduce immune responses and manufacturing time. By customizing cell sources and employing immune-matched biomaterials, you’re moving closer to creating organs that seamlessly integrate with your body, reducing or eliminating the need for immunosuppressants.
What Are the Main Regulatory Hurdles for Clinical Organ Bioprinting?
You need to navigate evolving regulatory hurdles, including establishing standards for manufacturing, sterility, and potency of bioprinted organs. You’ll face challenges in ensuring safety, consistency, and long-term functionality, as current frameworks aren’t fully adapted for complex biologics combined with devices. Additionally, addressing ethical concerns like equitable access and ownership, along with developing approval pathways for personalized, multi-component tissues, will be vital for clinical translation.
How Cost-Effective Will Future Bioprinting Solutions Be for Widespread Use?
Future bioprinting solutions will become more cost-effective as automation and AI-driven processes streamline manufacturing, reducing labor and material costs. As techniques improve, scaling up production and standardizing quality control will lower expenses, making organs more accessible. You’ll see decreased reliance on complex cell sourcing and faster regulatory approvals, further driving down costs. Over time, these innovations will make widespread use feasible, improving patient access and transforming transplant medicine.
What Ethical Issues Might Arise From Bioprinting Organs With Nanotech?
You could face ethical dilemmas that shake the very foundation of medical morality. Nanotech-enabled bioprinting raises concerns about equitable access—who gets these life-saving organs—and ownership of genetically or digitally designed tissues. You might also confront questions about consent, especially if organs are customized or enhanced. The potential for creating “designer” organs could lead to societal inequalities, prompting urgent debates on fairness, regulation, and the limits of human intervention.
Conclusion
As you explore the exciting evolution of organ printing with nanotech, remember the remarkable road ahead. By bridging breakthroughs in bioprinting, battling biological barriers, and balancing ethics, you’re part of a pioneering path toward perfecting transplant technology. With relentless research, resilient innovations, and responsible regulation, you can help turn this breathtaking breakthrough into a breathtaking reality. Together, you’re shaping a future where faulty organs fade, and fantastic, fully-functional transplants flourish.